Source: https://physics-and-radio-electronics.com/physics/laser/principlesofworkingofalaser.html
Laser and photon interact with atoms in three ways:
Absorbed radiation
Spontaneous emissions
Stimulating emissions
Absorbed radiation
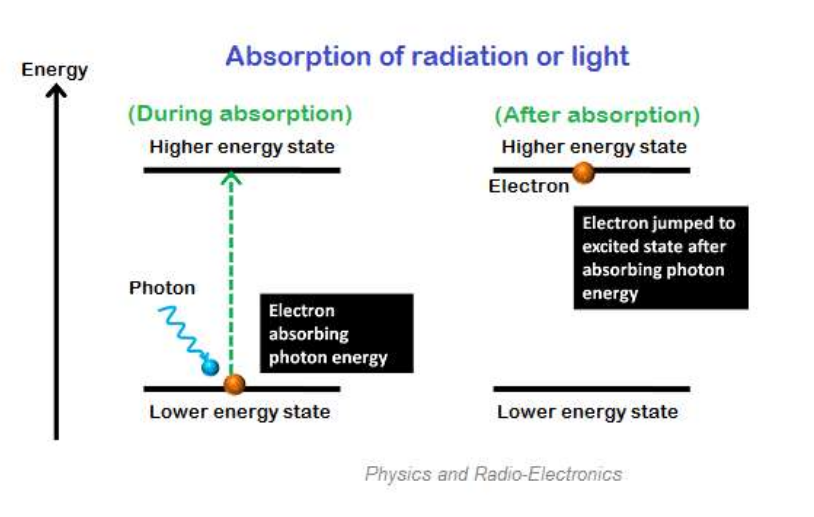
Absorption radiation is the process in which electrons absorb energy from photons underground to jump into higher energy levels.
The electron orbits very close to the atomic nucleus at a lower energy level. As the electron orbits, the energy level or lower energy state is higher as it moves further away from the nucleus. Electrons at lower energy levels require some additional energy to jump to higher energy levels. This additional energy is provided by various energy sources such as heat, electricity, or light.
Let's consider electrons with two energy levels (E1 and E2). E1 is the ground state or lower energy state of electrons and E is the energy state of excited electrons or higher energy states. The ground state of electrons is called low-energy electrons or base state electrons, while electrons in the excited state are called high-energy electrons or excited electrons.
Generally speaking, electrons in low energy states cannot jump into higher energy states. They need enough energy order to jump into higher energy states.
When photons or light energy equal the energy difference between two energy levels (E2-E1) are incident on an atom, the ground state electrons gain enough energy and jump from the ground state (E1) to the excited state (E2).
This absorption only occurs when the incident energy matches the energy difference between the radiation or photon and the two energy levels (E2-E1).
Spontaneous emissions
Spontaneous emission is the process by which excited electrons return to their ground state by emitting photons.
This electron can only stay in an excited state for a short time. The excited electrons that can remain in a higher energy state for a longer period of time (E2) are called excited lifetime electrons. The lifetime of electrons in the excited state is 10-8 seconds.
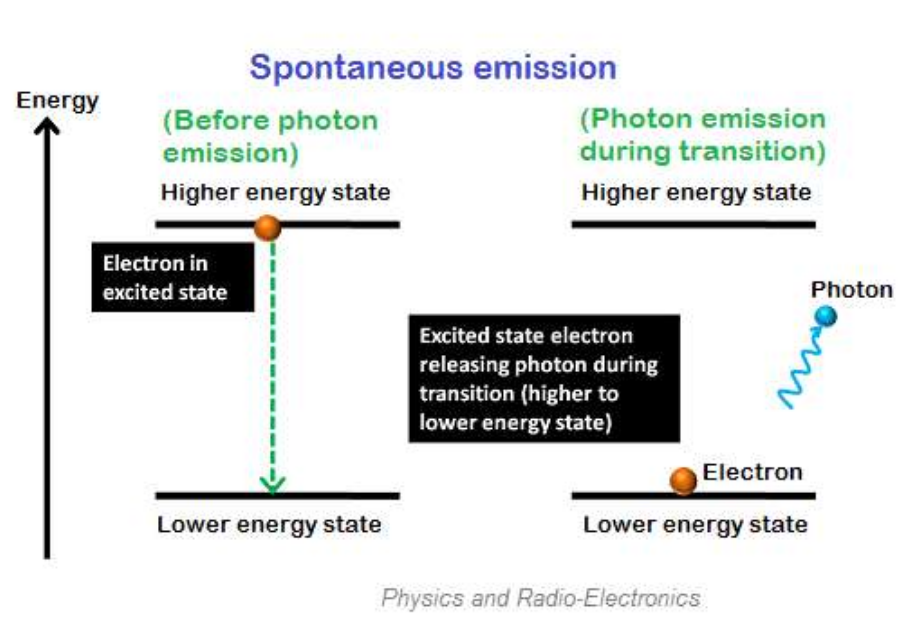
Therefore, after the brief lifetime of excited electrons, they will return in the form of photons by releasing energy to lower energy states or ground states.
In spontaneous emission, electrons naturally or spontaneously transition from one state (high-energy state) to another state (low-energy state), so the emission of photons also occurs naturally. Therefore, we cannot control when excited electrons will lose energy in the form of light.
The photons emitted during spontaneous emission constitute ordinary incoherent light. Non coherent light is a beam of photons with frequent and random phase changes. In other words, the flow direction of spontaneously emitted photons during the emission process is not completely the same as the incident photons.
Stimulating emissions
Stimulus emission is the process of incident photons and excited electrons forcing them back to the ground state.
When stimulated emission occurs, light energy is directly provided to the excited electrons instead of providing light energy electrons to the ground state.
Spontaneous emission and stimulated emission are not natural processes, but rather artificial processes.
During spontaneous emission, electrons in an excited state will remain there until the end of their lifecycle. After completing their entire lives, they return to the ground state light by releasing energy.
However, when stimulated, electrons are in an excited state and do not need to wait to complete their lifetime. Another selection technique is used to force excited electrons back to their ground state before their lifetime is completed. This technology is called stimulated emission.
When the event photon interacts with the excited electron, it forces the excited electron to return to the ground state. This excited electron releases its ground state energy in the form of light when it falls.
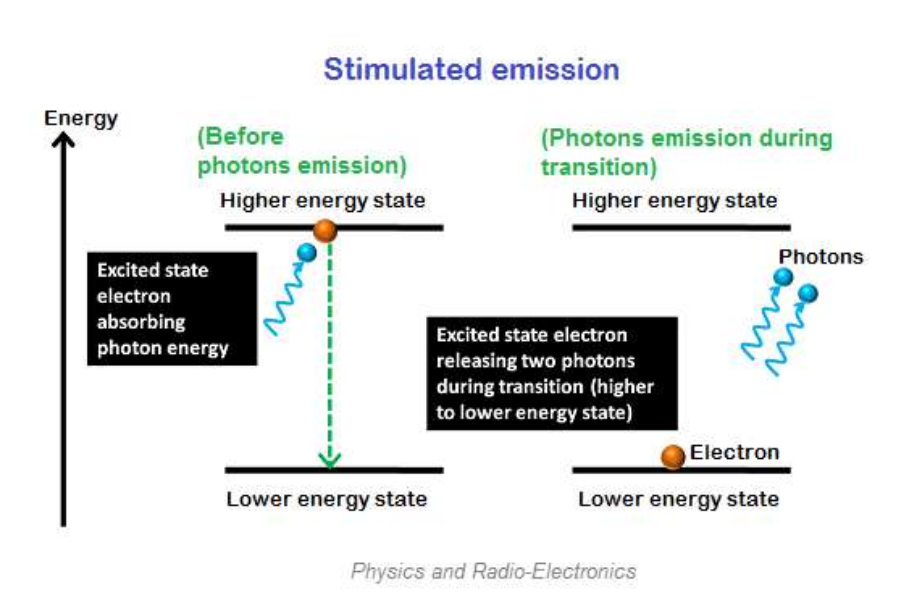
In stimulated emission, two photons are emitted (the other photon being emitted), one due to the incident photon and the other due to the excited energy releasing an electron. Therefore, two photons were emitted.
Compared to spontaneous emission, the emission process of this stimulus is very fast, and the emitted photons have the same energy, frequency, and phase when stimulated. Therefore, all photons propagate in the same direction when excited.
The number of photons emitted by this excitation depends on the number of electrons at higher energy levels or the excited state and incident light intensity.
It can be written as: the number of emitted photons, the number of electrons in the alpha excited state, and the incident light intensity. The flow direction is not exactly the same as the incident photons.
*Disclaimer: The content of the article is the author's personal opinion. Reproduction on the website is only intended to convey a different viewpoint and does not represent the company's endorsement or support of that viewpoint. If you have any objections, please feel free to contact us.